Reports of massive fish kill events in lakes across Bengaluru come up worryingly often every year after the first monsoon showers. This year was no different. Ulsoor, Basavanapura, Muthanallur and Bhattarahalli are some of the lakes in which dead fish were found floating over the last few weeks.
What is causing this morbid scene year after year in a city famed for its many water bodies? Pollution is one culprit. What are the other factors involved? How exactly do these interact and impact lake ecosystems? And what needs to be focused on to prevent such events?
To understand the whole story, we first need to explain what dissolved oxygen (DO) is and what causes DO levels in the lakes to rise and fall.
Effects of pressure, temperature and salinity on lakes
Water is a universal solvent. All gases dissolve in water in various proportions. For oxygen, the dissolved concentration depends on pressure, temperature and salinity. DO levels bear an inverse relationship with temperature and salinity; and a direct relationship with pressure. This means as the temperature rises or if the water becomes more saline, the amount of oxygen dissolved in the water dips; but as the pressure increases (like it does when you travel from high to lower altitudes), DO levels in water rise.
The maximum amount of DO water can hold depends on all three factors and is called saturation DO. When pressure and salinity are kept constant, the saturation DO level in water is dictated by the temperature. For example, the saturation concentration of DO in water at 0 degrees is 14 mg/l (milligrams per litre) or parts per million (ppm), whereas at 100 degrees it is 0 mg/l. This is a standard measurement.
Factors that prompt deoxygenation or reoxygenation
However, at a given temperature, the actual DO level in a water body could be lower than the standard saturation level. Various factors such as the breakdown of organic matter, presence of chlorophyll-a, salinity and other organic sediments cause deoxygenation, i.e. the removal of dissolved oxygen in the water.
When nutrients, primarily phosphorous, enter water bodies, they bind to sediments in the water and get deposited on the lake bed. The anaerobic conditions (lack of oxygen) at the bottom of the lake cause phosphorus to dissolve, making it available for algae. This is called ‘internal loading’ of nutrients (external loading is when nutrients enter a water body through sewage lines or drains or the land around it). Therefore, the higher the nutrient levels in lakes, the more feed there is for the algae.
The algae then multiply, causing the process called eutrophication when algal blooms cover the surface of lakes, blocking light and killing flora below. The dead algae contribute to lake sediments fuelling the feedback loop for internal loading of nutrients and maintaining high rates of deoxygenation.
Oxygen also dissolves in the water from the atmosphere through reaeration processes, which are prompted by the speed of water flow and the wind.
The difference between the saturation DO and actual DO levels is called the DO deficit. The figure below lists the factors affecting deoxygenation and re-aeration rates and thus determine the DO levels in surface water bodies.
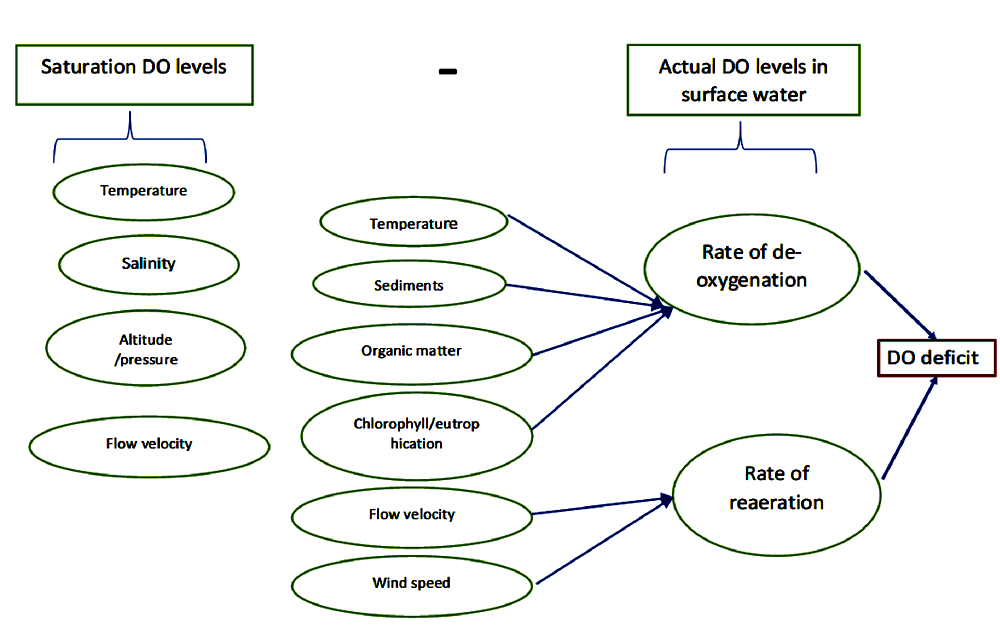
Read more: Why fish die in Bengaluru lakes, and what you can do to prevent it
Managing ‘critical DO levels’ in lakes
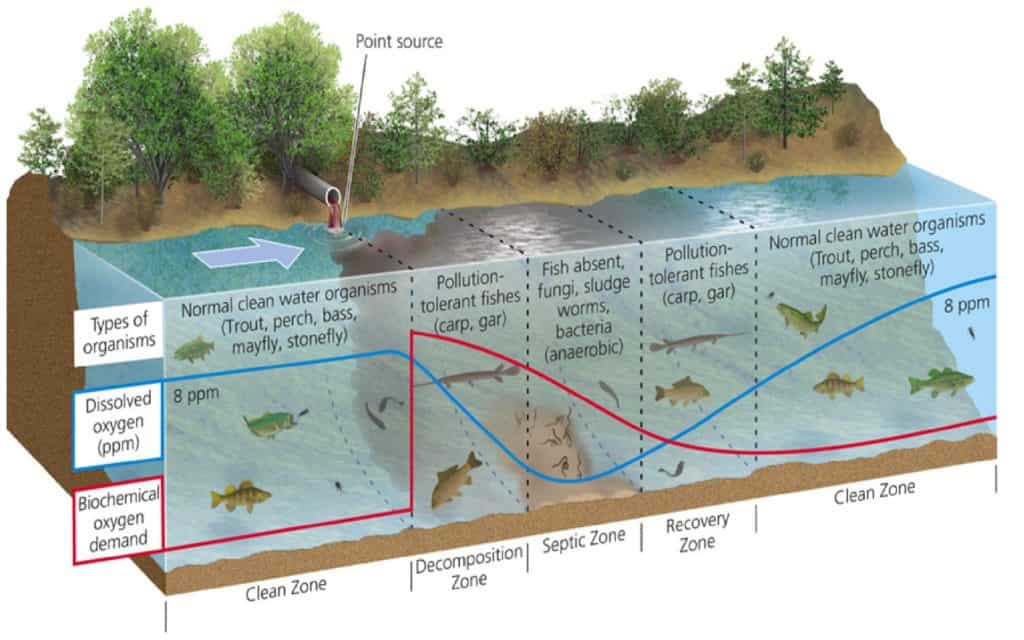
Now that we’ve gone over the basics of DO levels and what influences them, we can delve into what happens in aquatic environments. Immediately downstream of an effluent discharge point, the deoxygenation rate is greater than the reoxygenation rate, which means the water is losing oxygen faster than it can be replenished.
As the water flows farther downstream, the decomposition of organic matter reaches a point where deoxygenation equals the reoxygenation rate. At this point, the water body experiences minimum DO levels, also known as critical DO (the septic zone in the image below). Beyond this point, reoxygenation soon outpaces the deoxygenation rate and thus the DO levels rise farther downstream from the source of effluents.
The figure below illustrates the impact of wastewater discharge on the DO levels in water bodies at different points.
Mapping the DO sag curve in Jakkur lake
The DO dynamics in water bodies is termed the DO Sag Curve. We used this approach to understand large-scale fish kill events in several lakes across Bengaluru. We measured DO levels along the flow of water (inlet to the outlet) to map the DO Sag Curve at Jakkur lake in the north of the city, which has witnessed frequent fish kill events after the first pre-monsoon showers. We carried out the survey on May 9 this year.
The figure below shows the DO levels along the length of the lake. The third point listed in the map shows the minimum DO levels of 1.12 mg/l, much below the levels required for fish survival. Only two points (7 and 9) recorded slightly above 4 mg/l.
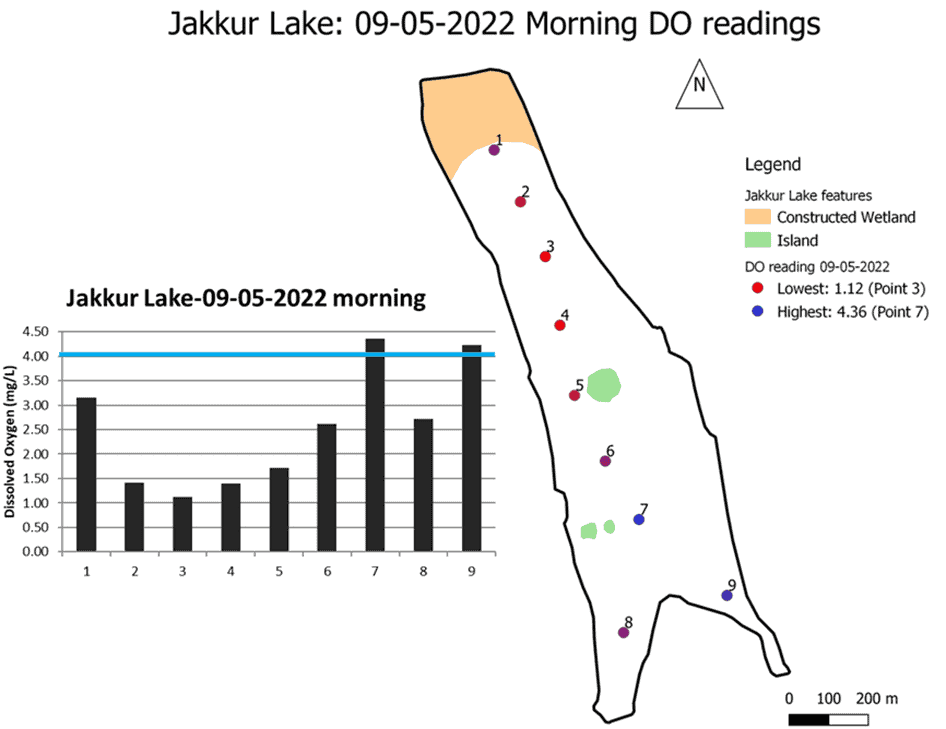
Read more: Polluted lakes, poor waste management contaminating Bengaluru’s groundwater
Change of seasons, weather conditions affect lake DO levels
Why do fish deaths spike in the summer and coincide with pre-monsoon showers? As explained in the beginning, temperature plays a central role in influencing DO levels. The transition from winter to summer season causes an approximately 10 degrees rise in ambient temperature. These higher temperatures increase the rate at which oxygen is depleted from the water body which in turn promotes algal growth.
Moreover, the first rains bring additional pollutants from non-point sources (i.e. more widespread run-off and not effluents from sewage lines alone), providing even more organic matter for microorganisms to break down by consuming the oxygen dissolved in water. In addition, low wind speed during the summer and pre-monsoon weeks significantly reduces the rate at which oxygen is added to the lake, thereby leading to large-scale fish kills.

It is possible to reduce mass fish death incidents
We can’t alter how nature operates, i.e. factors such as temperature and wind speed that affect oxygen levels in the water. But fish kill events can be prevented by two important steps:
- Managing nutrients (i.e., nitrogen and phosphorus) and organic matter loading in the inflows and within the water body; and
- Artificial aeration.
Lakes perform an important ecosystem service in cities, which is why it is critical that measures are taken to preserve the health of water bodies. Deploying effective nature-based solutions in conjugation with secondary wastewater treatment plants will reduce external loading. Combining nature-based solutions such as floating islands and food chain management will help manage internal loading by physically removing nutrients and organic matter from the lake.
Well explained and written. Typical Priyanka. This is good, but requires more depth, may be as a series. I observe that While reading through somewhere ‘pressure’ as a factor seized to be analysed. Further, lakes do not have uniform temperatures, atleast lakes with biodiversity and natural water flows. Temperature difference within a lake has been a factor. The shelter areas for aquatic life are missing now. Water level in our lakes used to go down and rise, as per season. Presently, in cities, these lakes are perennially loaded. Lakes usually created a micro-climate, with temperature difference within a boundary and outside. With concretisation around them, this microclimate would also be under stress from temperatures and radiation from outside. We need to explore how bio-resources can help in sustaining DO levels. For my clarification, does COD levels impact DO levels? If so, how?